SERCA2a Activators
SERCA2a PORTFOLIO WITH POTENTIAL ORAL ADMINISTRATION FOR CHRONIC DOSING
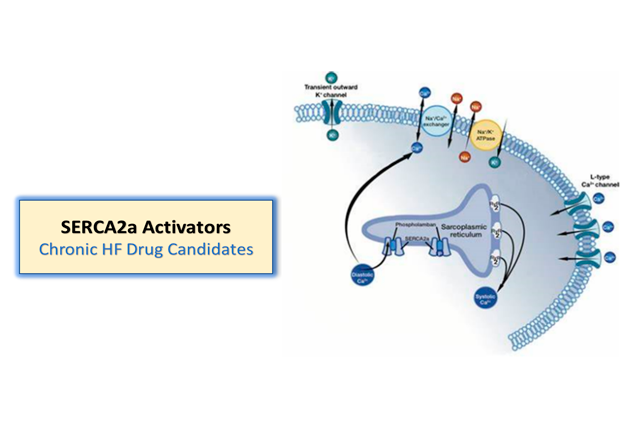
One of Windtree’s objectives is to develop a multi-asset franchise anchored around its ability to activate SERCA2a in a unique manner. With istaroxime representing an important proof of concept for this approach, Windtree has multiple follow-on oral and intravenous SERCA2a activators in preclinical development. These drug candidates may position Windtree to potentially treat not just those suffering from acute heart failure, but also potentially provide chronic therapy upon the patient’s hospital discharge.
WINDTREE HAS TWO TYPES OF SERCA2a ACTIVATORS
Pure SERCA2a Activators
The Pure SERCA2a activators are devoid of any Na/K pump inhibitory activities. These compounds are intended to be oral (with IV possible) therapies for AHF and/or CHF and have recent patent filings. These candidates also represent a potentially attractive approach for heart failure with preserved ejection fraction (HFpEF) – an underserved population that represents nearly half of all heart failure. This family of the SERCA2a Activators has received its granted patent for composition of matter from the European Patent Office and from the US Patent and Trademark Office.
Dual Mechanism SERCA2a Activators
Like istaroxime, these compounds have a dual mechanism of action as SERCA2a activators and Na/K pump inhibition. These product candidates are intended to be oral and IV therapies for AHF and/or CHF and could be utilized as a “fast follow on” to istaroxime in AHF and as oral chronic outpatient treatment upon discharge.
This family of the SERCA2a Activators has received its granted patent for composition of matter from the European Patent Office and from the US Patent and Trademark Office.
Windtree is advancing all our heart failure programs and will be engaged in potential partnering and/or licensing discussions to foster development.