company OVERVIEW
WELCOME TO WINDTREE THERAPEUTICS
Windtree Therapeutics is a biopharmaceutical company focused on oncology and cardiovascular treatments intended to address markets with significant unmet need (OTCID: WINT)
First in class, novel asset istaroxime has demonstrated positive efficacy and an attractive profile compared to currently available rescue medications in three Phase 2 global studies, highlighted by improvements in cardiac function and increases in blood pressure with favorable renal function profile
Istaroxime is in Phase 2 clinical development for cardiogenic shock and acute heart failure; platform also includes next generation oral, SERCA2a activators in preclinical development
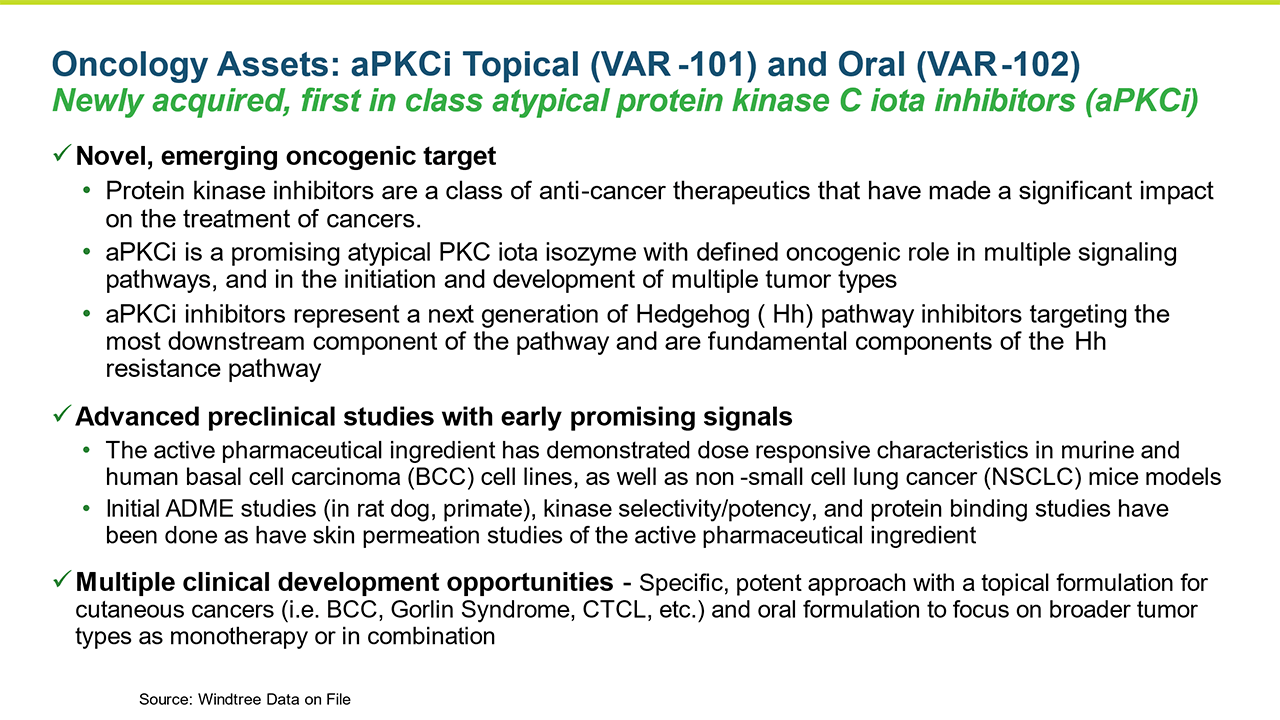
Newly acquired first in class, novel, protein kinase C iota inhibitor oncology platform with potential topical and oral formulations creates significant opportunity that we plan to advance this year
Global and regional license deals in place with a priority focus on potential additional global license or strategic transaction with new partner for cardiovascular assets
Lean, capital efficient operation led by a highly experienced management team in oncology and cardiovascular development, deals and commercialization
Istaroxime in Cardiogenic Shock
In May 2022, Windtree reported our positive Phase 2 study of istaroxime in early cardiogenic shock (SEISMiC study) results. The study’s positive primary endpoint and results have paved the way for our clinical development to potentially have istaroxime Phase 3 ready for cardiogenic shock.
After these positive data results, Windtree has prioritized istaroxime in cardiogenic shock as our focus among the cardiovascular assets. We made this decision based upon the data and these drivers of opportunity and potential commercial value:
- Significant opportunity for Istaroxime to make a difference:
- ~20-30% mortality in classic shock and high morbidity
- Very long average length of hospital stay (~19.5 days1) means high cost of hospital care (estimated >$175k2) and creates opportunity for pharmacoeconomic benefits
- Currently available pharmacologic treatments have undesirable side effects and can result in poor outcomes
- Lack of competition in development or active competition in the market
- Attractive $1.25B valuation of market potential versus time and cost of development supports potential deals
Cardiogenic shock is a severe presentation of heart failure characterized by low blood pressure and inadequate blood flow to vital organs (hypoperfusion) accompanied by congestion and high filling pressures of the heart.
There is potential for a relatively fast and less expensive development and regulatory pathway for istaroxime.
Windtree’s Unique Approach with SERCA2a Activation
Windtree’s heart failure platform includes follow-on preclinical SERCA2a Activator assets as well.
Dual mechanism SERCA2a Activators: This class of drug candidates have a similar MOA to istaroxime. The first mechanism strengthens the contraction of the heart and the second mechanism promotes the relaxation of the heart’s ventricles so they can fill with more blood in between the heart beats. This group may be well suited for study in both the hospital inpatient area for acute HF where it could be administered as an IV and in the outpatient area as a potential oral pill formulation after a patient is released from the hospital. This group could be developed as a “fast follow on” to istaroxime in acute decompensated heart failure but offer the potential benefit of chronic dosing following patient hospital discharge.
Pure SERCA2a Activators: This group’s mechanism of action focuses on the relaxation of the ventricles to fill more with blood in between heart beats. The group may be well suited for study in chronic heart failure as an outpatient in heart failure with preserved ejection fraction or HfpEF. This is an area highly underserved in CV disease.
Windtree’s Oncology Pipeline: aPKCi Inhibitors
______________________________________________________
1 US Hospital Claims Data, 2022
2 Healthcare.gov, Department of Health & Human Services, estimated from average cost of hospital stay
