ISTAROXIME
ISTAROXIME INTRODUCTION
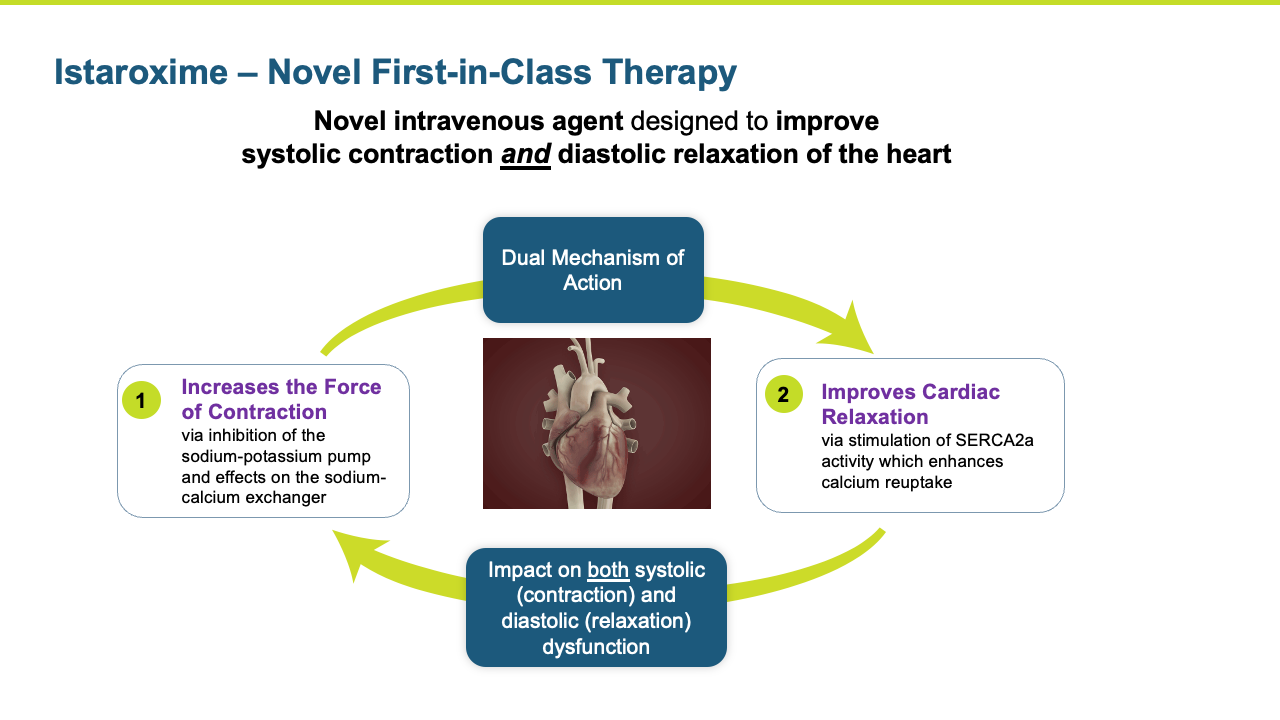
Istaroxime is a first in class, novel intravenous agent designed to improve systolic contraction and diastolic relaxation of the heart.
Through four Phase 2 global studies, lead asset istaroxime has consistently demonstrated a highly unique and attractive profile
-
It is the only acute heart failure or shock drug that has demonstrated both significant improvement in cardiac function of a failing heart, as well as rapid and significant improvement in blood pressure, with what so far has been a differentiating safety profile (avoiding many of the use-limiting side effects of existing therapies)
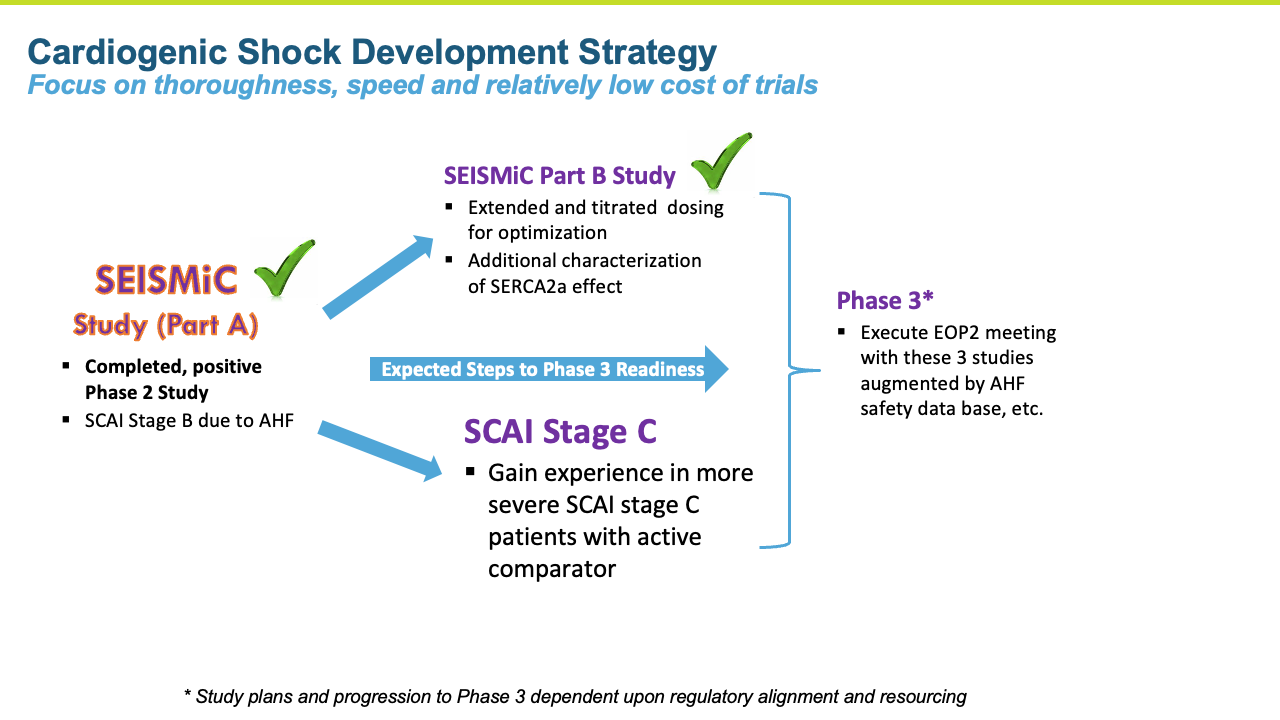
Recent positive Phase 2 study with istaroxime in early cardiogenic shock (ECS), SEISMIC A and B studies, paves the way for what we believe is a relatively fast and less expensive developmental and regulatory pathway
ISTAROXIME CLINICAL DEVELOPMENT EXPERIENCE – EARLY CARDIOGENIC SHOCK
Cardiogenic shock is a severe presentation of heart failure characterized by very low blood pressure and hypoperfusion accompanied by high pulmonary capillary wedge pressure and decreased urine output. Cardiogenic shock is an area of extreme unmet need with no satisfactory pharmacologic interventions to reverse the condition, thus it has a high associated mortality and morbidity.
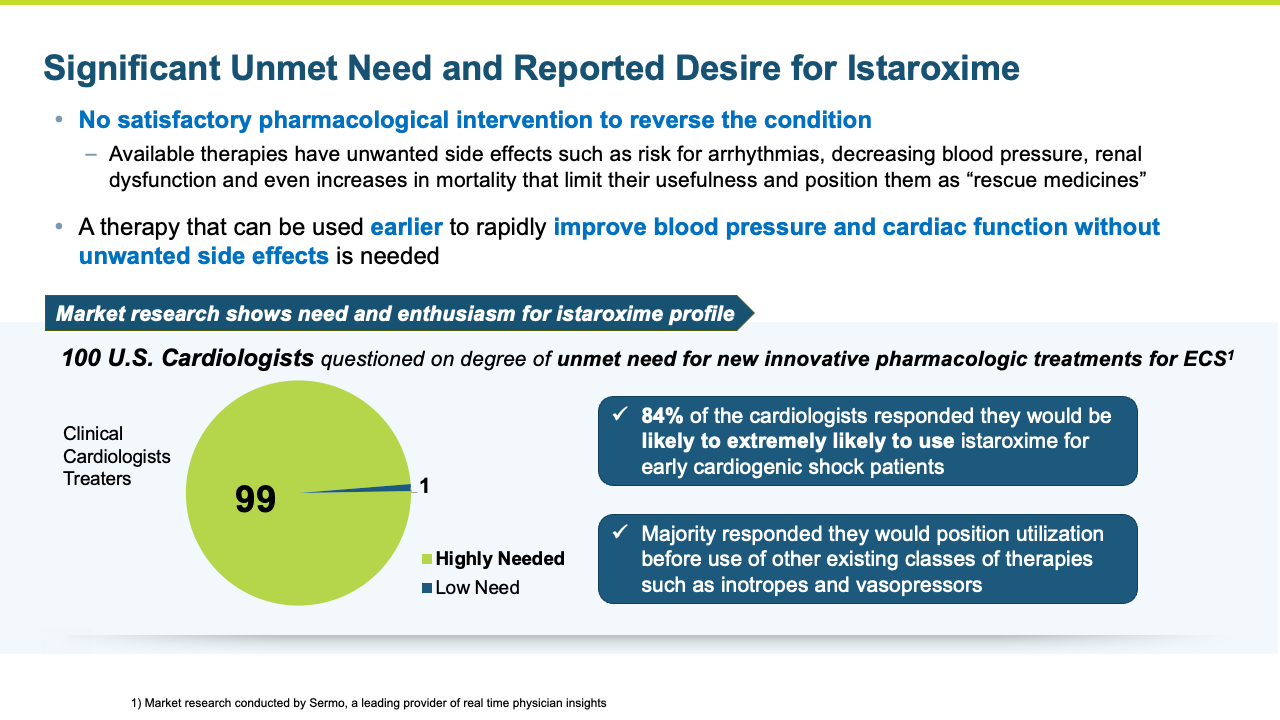
Istaroxime has the potential for an opportunity to address a significant unmet need in cardiogenic shock.
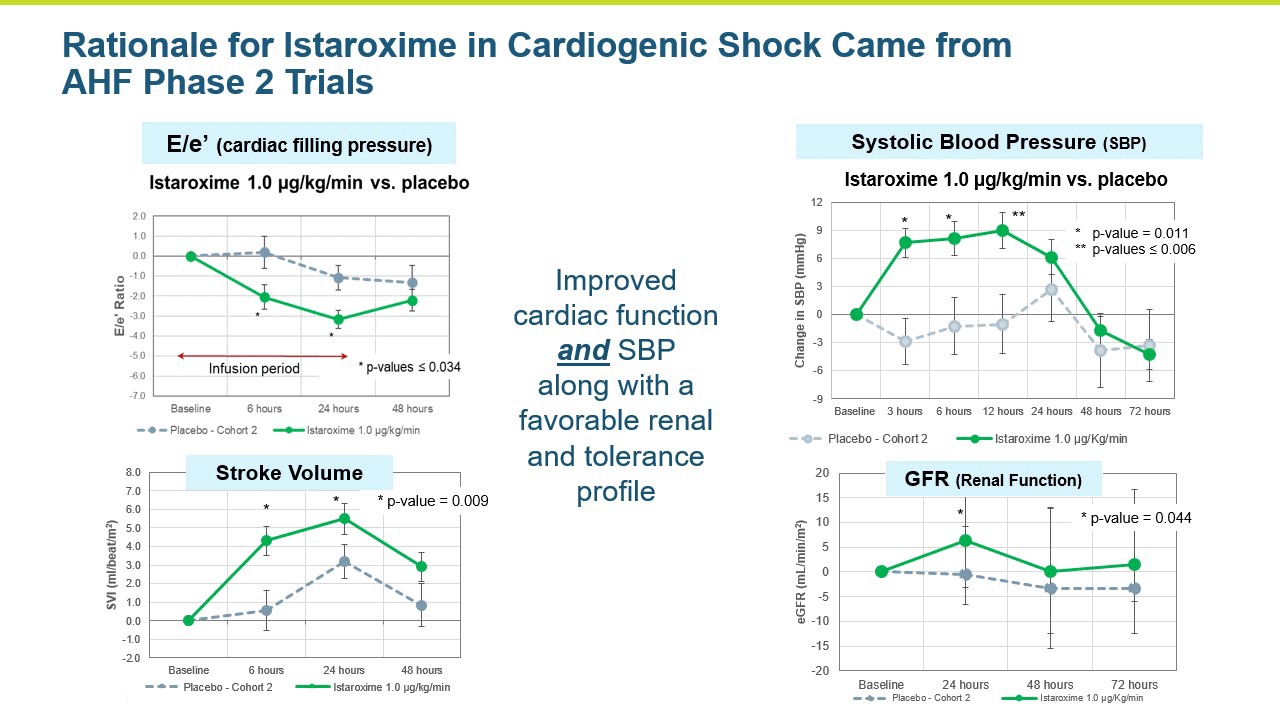
A central question that Windtree had about istaroxime in cardiogenic shock was whether it could increase systolic blood pressure in these very sick patients in a clinically meaningful manner. Systolic blood pressure increases were seen in the AHF Phase 2 studies.
Istaroxime Studies SEISMiC Part A and B – Early Cardiogenic Shock
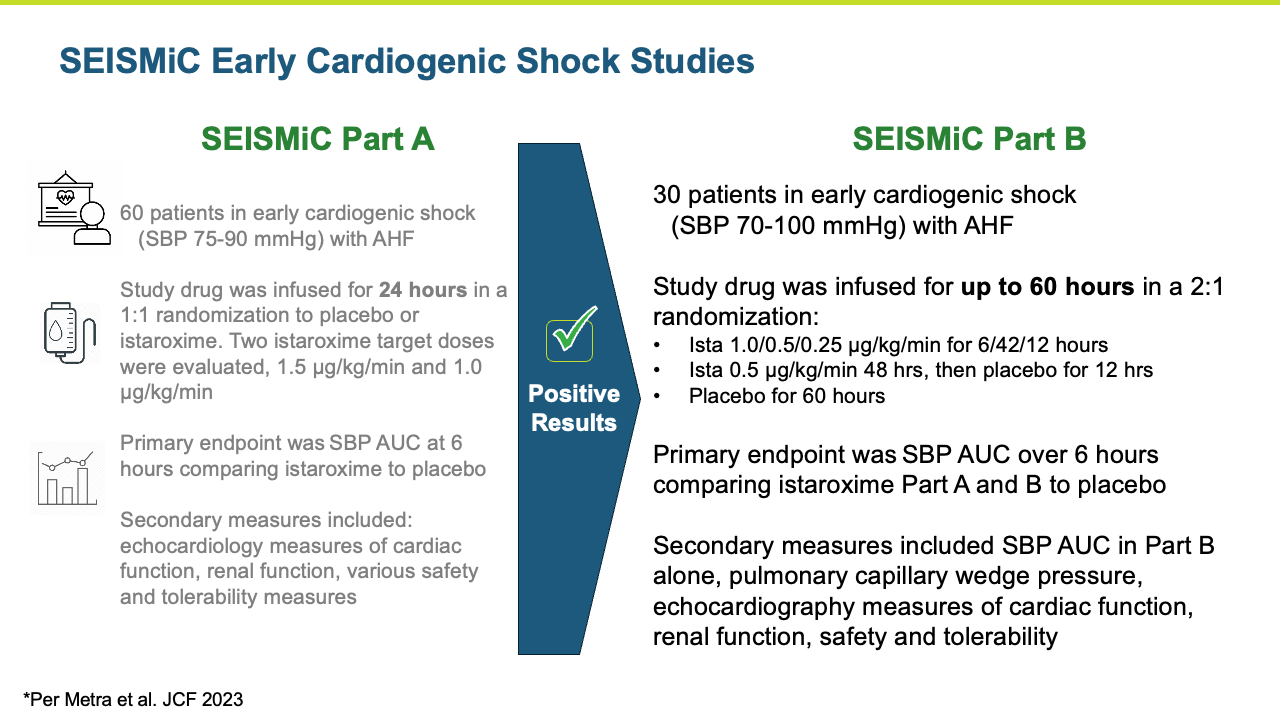
SEISMiC A
The SEISMiC Phase 2 Part A study in early cardiogenic shock is an international, randomized, double blind, placebo- controlled study enrolling 60 patients with Society for Cardiovascular Angiography & Interventions (SCAI) stage B early cardiogenic shock due to severe heart failure with systolic blood pressures (SBP) between 75-90 mmHg. Study drug was infused for 24 hours in a 1:1 randomization to placebo or istaroxime. Two istaroxime doses were evaluated, 1.5 μg/kg/min in the first group and 1.0 μg/kg/min in the next group. These groups were combined for analysis and compared to placebo. The primary endpoint was the difference in SBP area under the curve over six hours after initiating the infusion. Secondary endpoints included characterization of blood pressure changes over 24 hours, various assessments of systolic and diastolic cardiac function, assessment of renal function and measures associated with safety and tolerability.
The study was very successful. Below are the results:
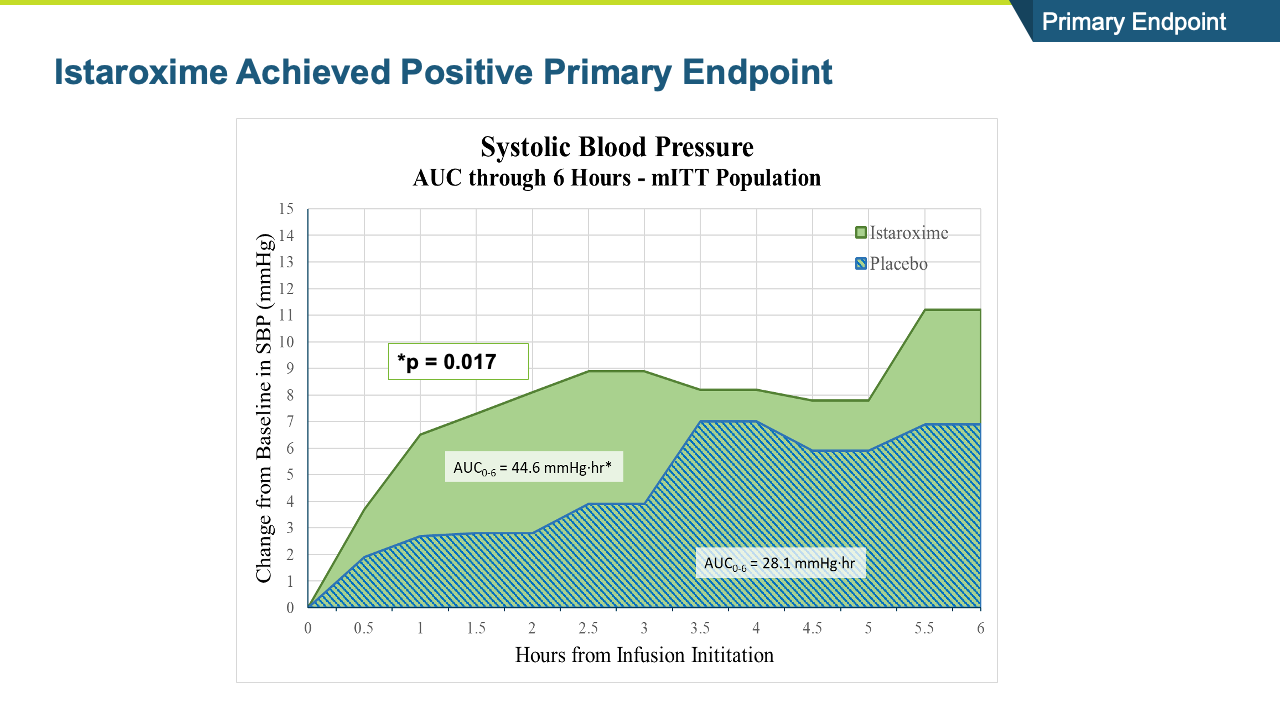
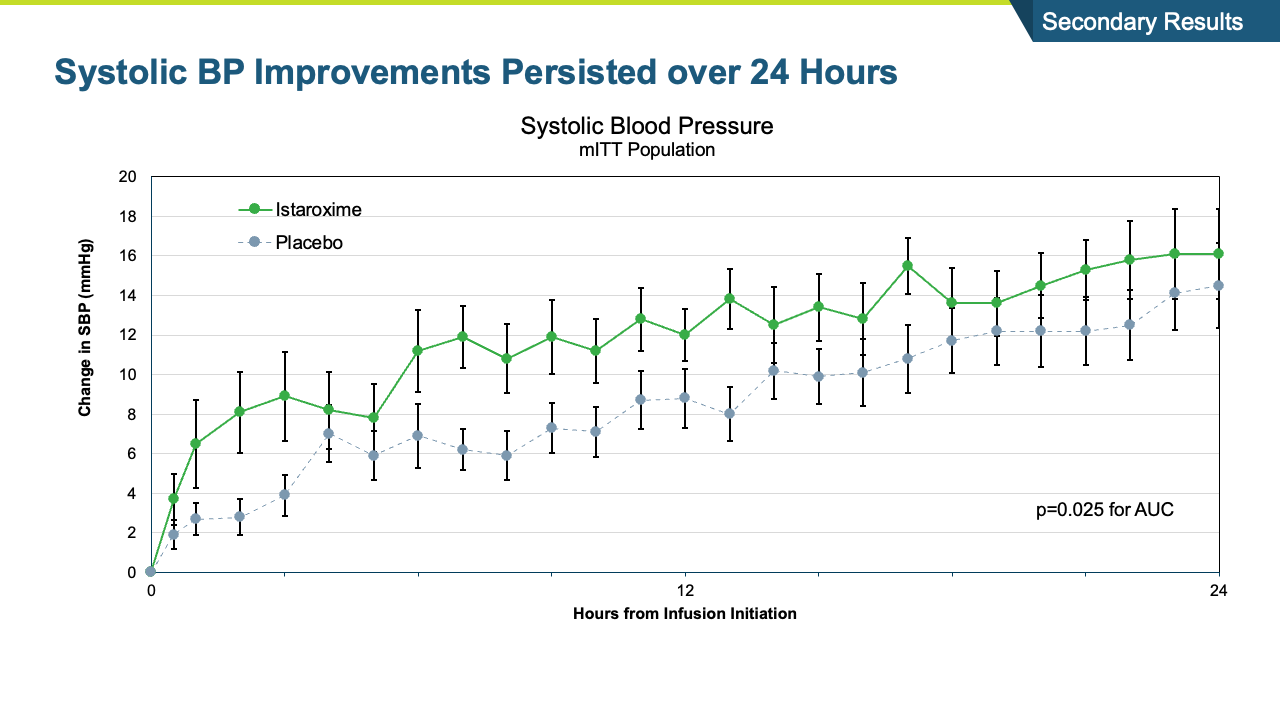
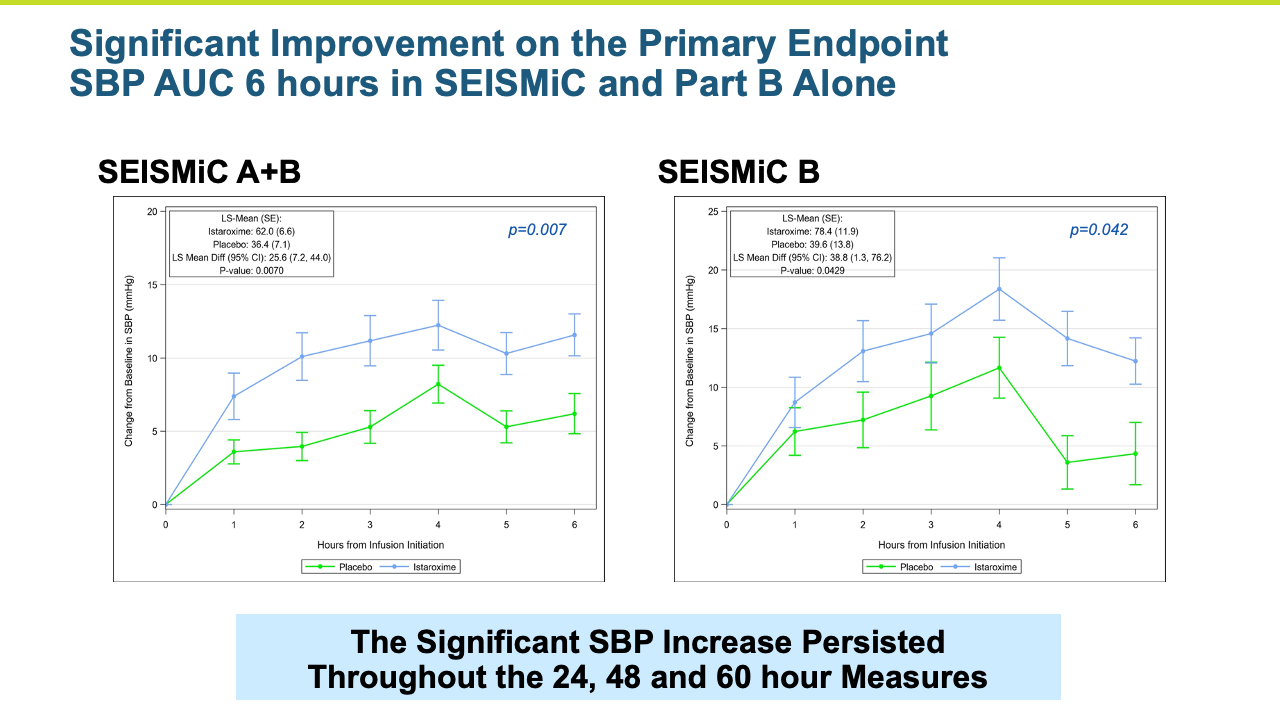
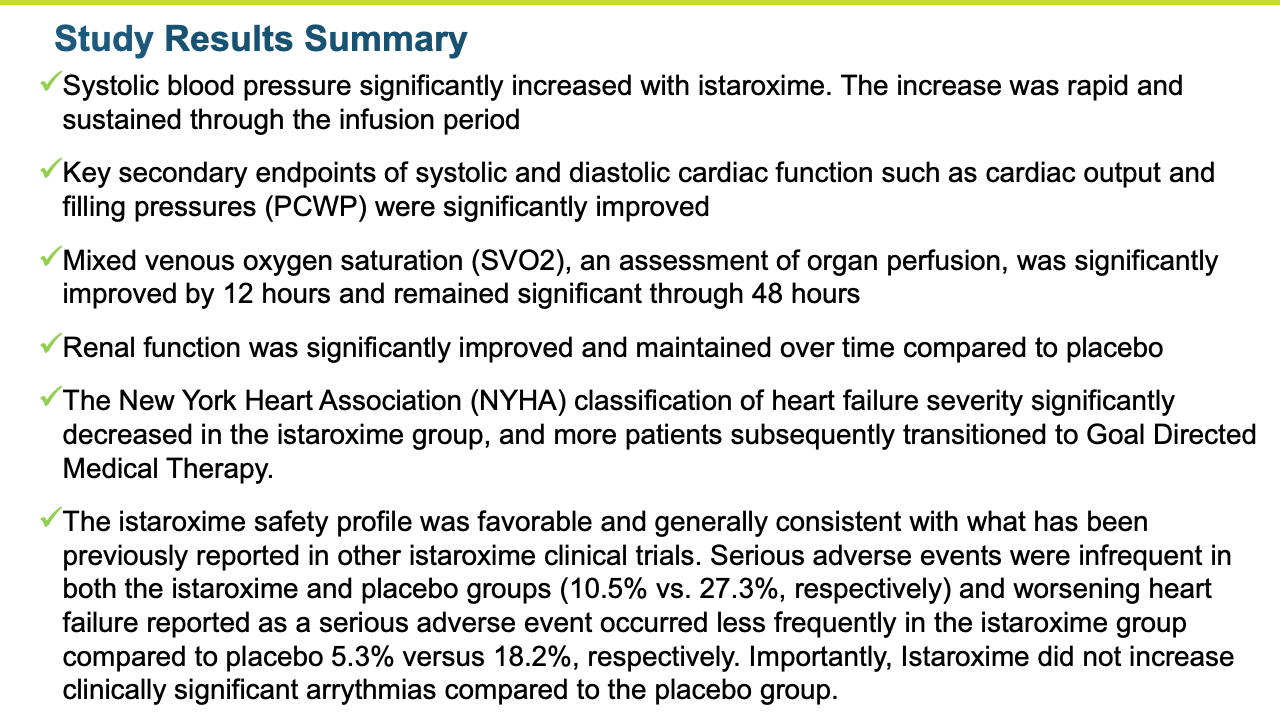
- The study met its primary endpoint in SBP profile over six hours, with the istaroxime treated group performing significantly better compared to the control group (p =0.017). The improvement persisted through the 24-hour SBP profile measurement which was also statistically significant (p= 0.025).
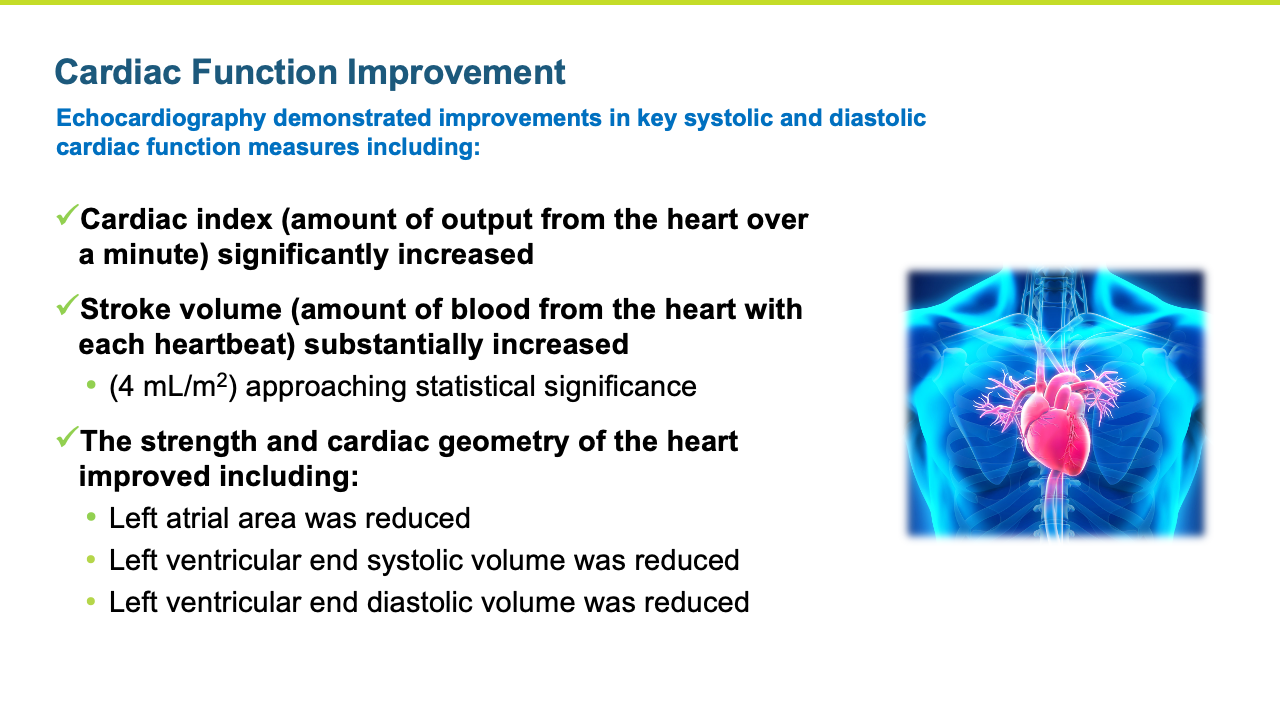
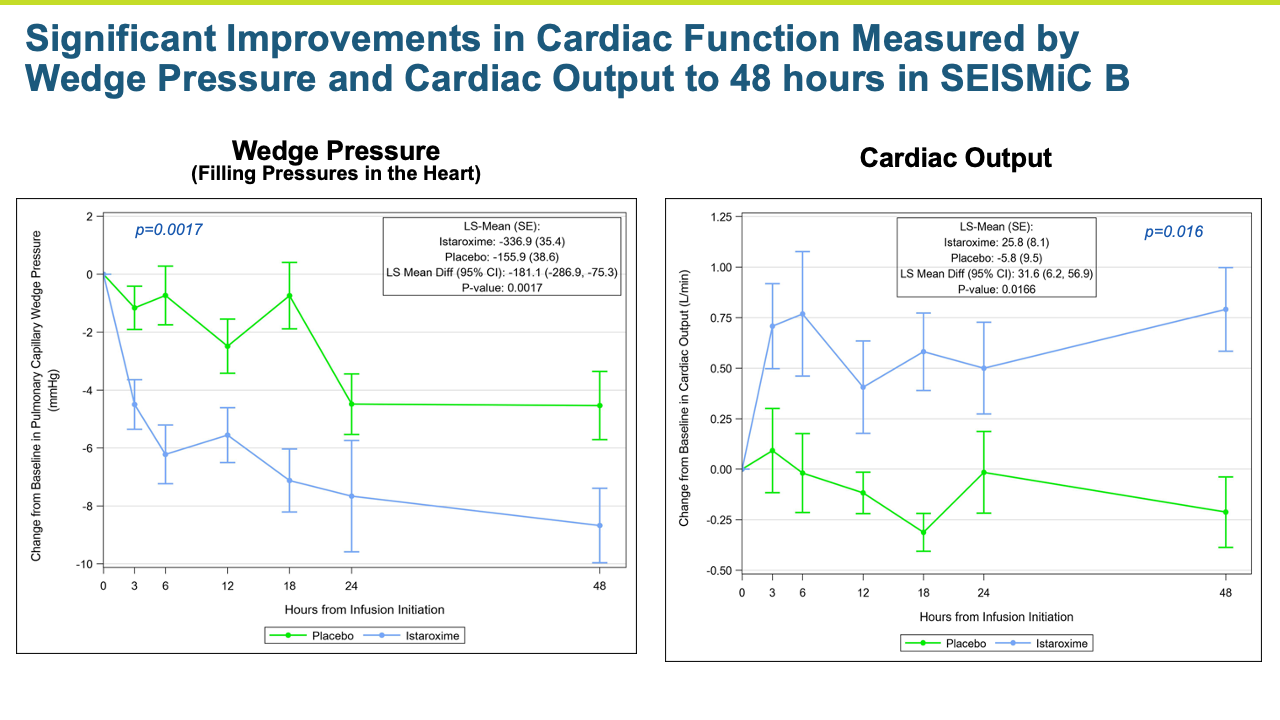
- The improvements in blood pressure were accompanied by a significant improvement in cardiac function as reflected by an increase in cardiac index compared to the control group (p = 0.016). Patients treated with istaroxime also experienced a substantial increase in stroke volume (the amount of blood pumped from the heart with each contraction).
- Several other key secondary echocardiographic parameters were significantly improved including left atrial area and left ventricular end systolic volume. Left ventricular end diastolic volume was also decreased with treatment.
- SBP was maintained post-infusion through the last measure at 96 hours
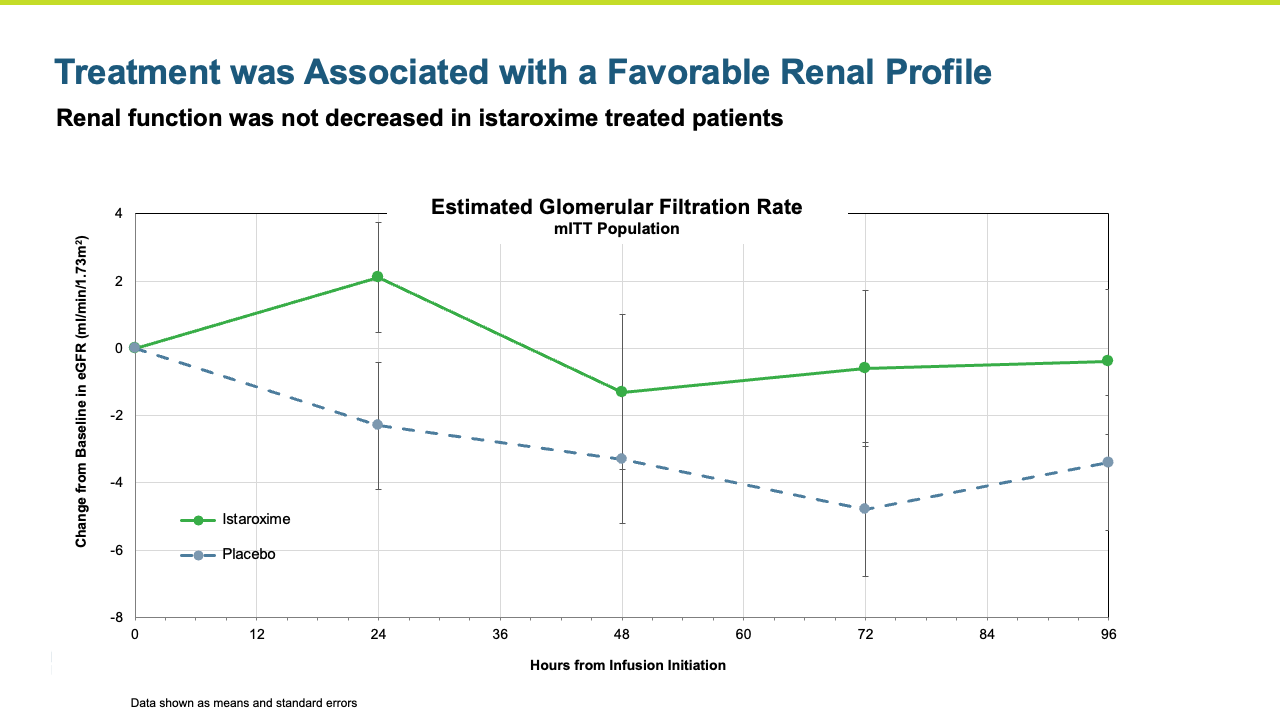
Renal function was maintained in istaroxime treated patients.
- Istaroxime was generally safe and well tolerated with the 1.0 ug/kg/min dose group producing a numerically greater increase in systolic blood pressure and a more favorable safety and tolerability profile than the 1.5 µg/kg/min dose group.
Summary:
- Systolic blood pressure significantly increased within the first 6 hours of initiating the infusion (p=0.017) and the increase was maintained throughout the 24-hour infusion (p=0.025)
- SBP increases were rapid within the first hour and sustained through the 96-hour post-infusion measure
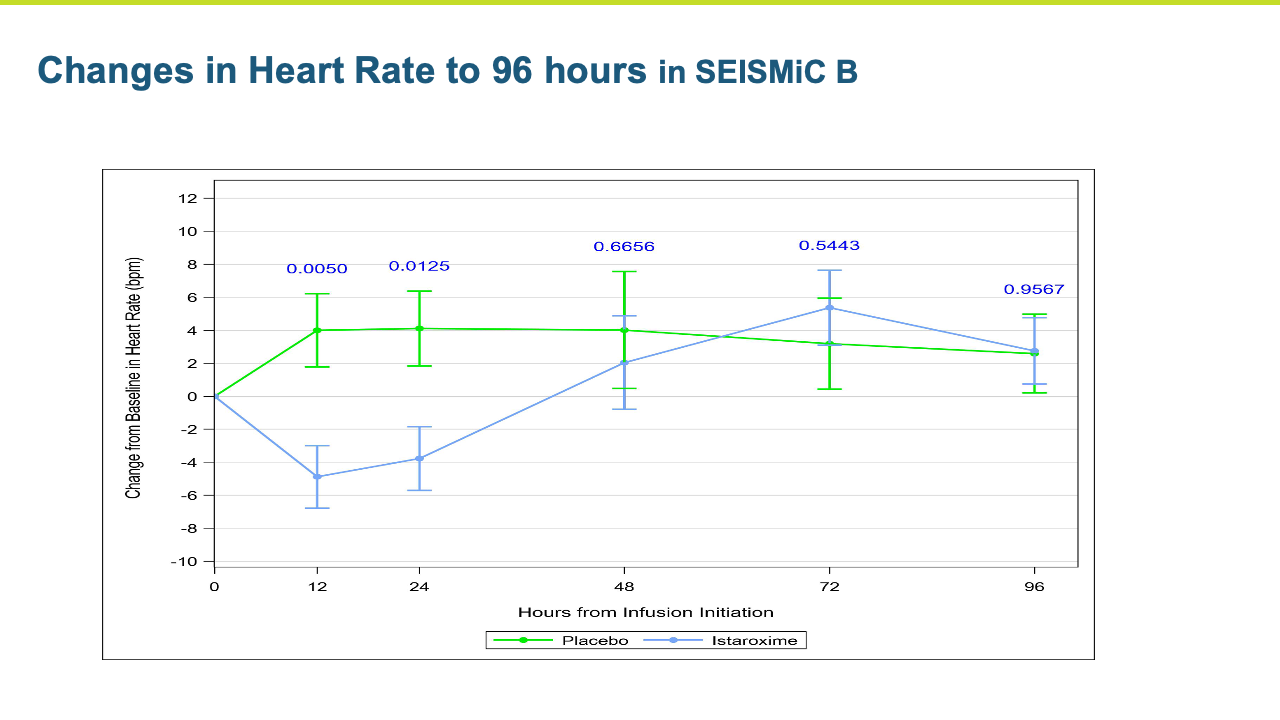
- Key secondary endpoints of systolic and diastolic cardiac function and performance were improved
- Renal function was maintained
- SEISMiC provided valuable information for optimizing our dose moving forward
- These data substantiate and advance the rationale for istaroxime as a potential treatment for cardiogenic shock and support the existing data from the program in AHF
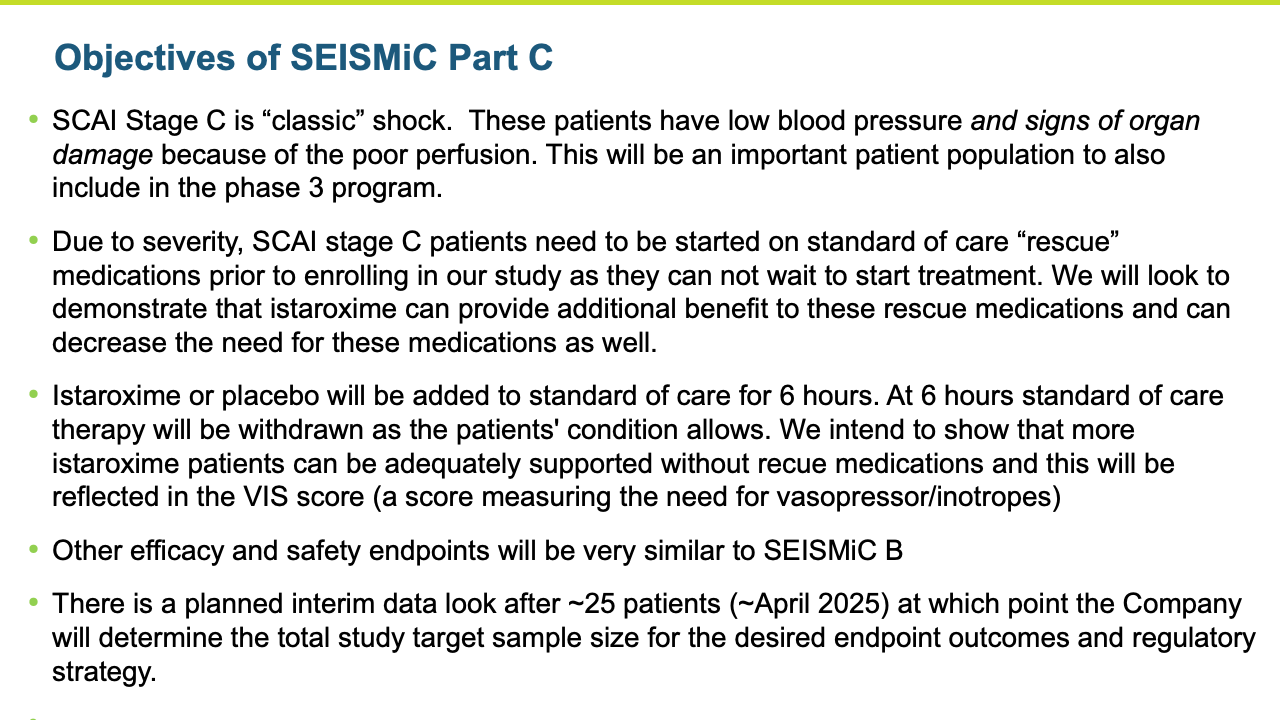
SEISMiC B
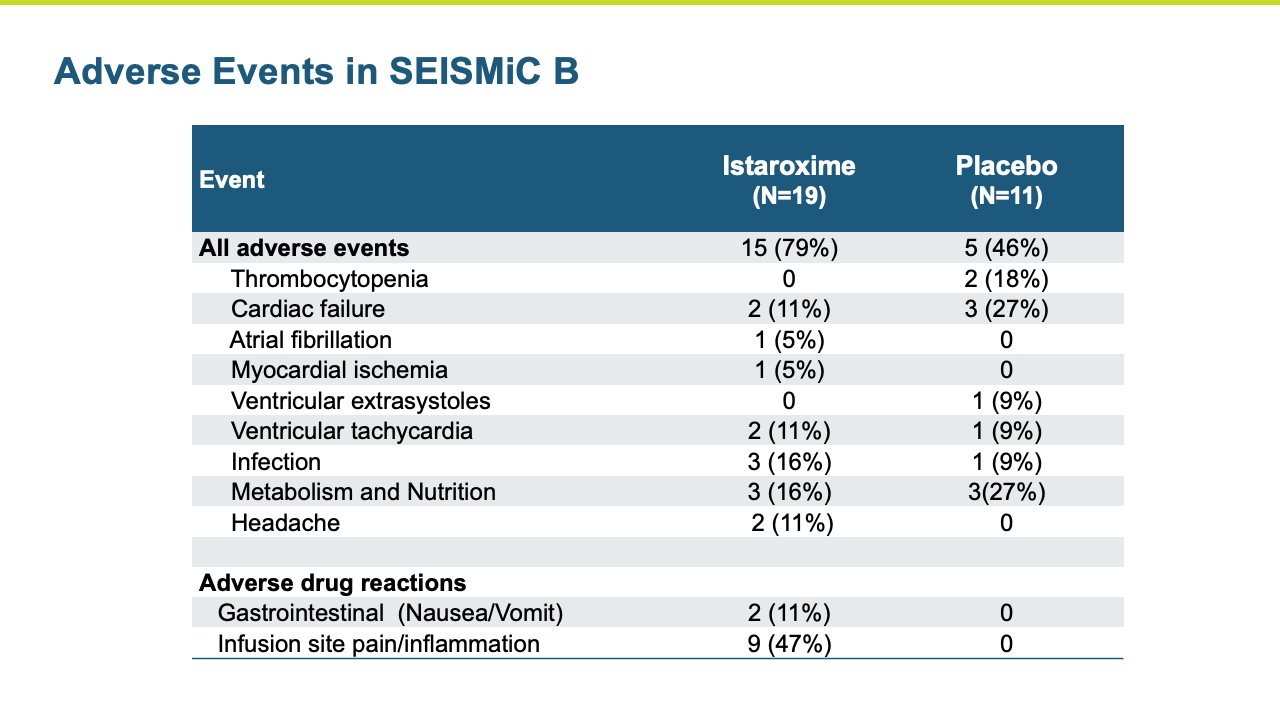
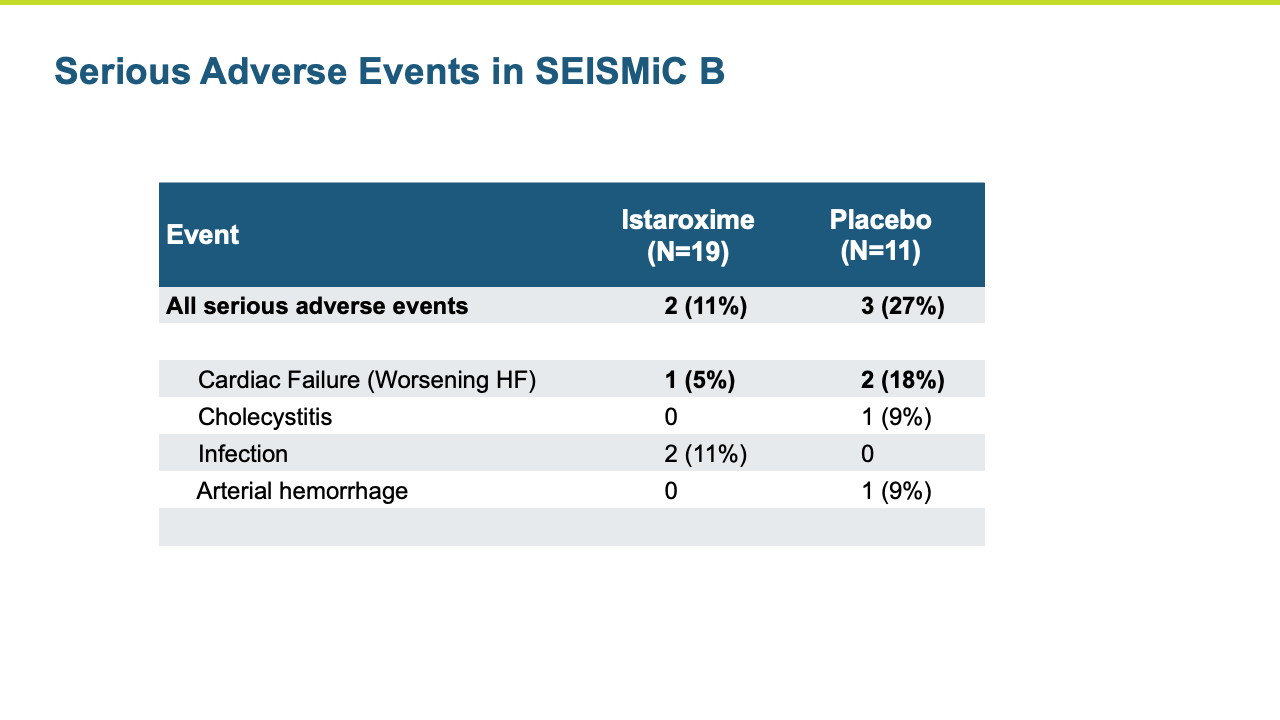
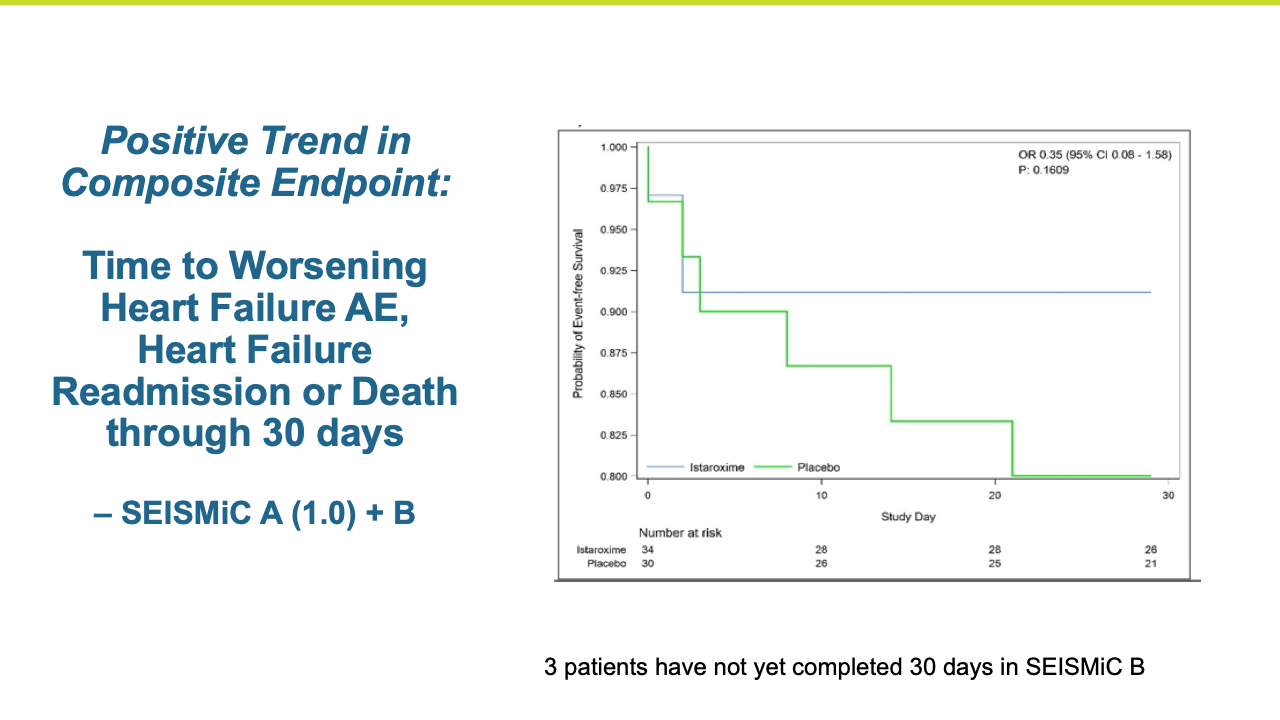
The Phase 2b SEISMiC Part B Extension Study in early cardiogenic shock (SCAI Stage B) randomized 30 subjects and was conducted in the United States, Europe and Latin America (3 subjects have been discharged but have not completed their 30-day visit). The study is focused on istaroxime’s observed ability to correct low blood pressure and to improve cardiac function and other parameters over 96 hours of close monitoring with the final visit at 30 days. The results build upon the positive results reported previously in the Phase 2b SEISMiC Part A study. The Part B study included hospitalized patients with SCAI Stage B cardiogenic shock with persistent hypotension due to acute heart failure and evaluated two different dose regimens of istaroxime compared to placebo. Patients in the active treatment groups received infusions of istaroxime for up to 60 hours, with one group receiving a decreasing istaroxime dose over time and the second group receiving a constant istaroxime dose. The study tested an extended dosing duration of istaroxime compared to previous studies, where treatment was limited to 24 hours, to determine the potential for additional benefit and, along with dose titration, to determine the optimal dosing regimen for an anticipated late-stage Phase 3 clinical trial. For the primary endpoint evaluating blood pressure, subjects from Part A and Part B of SEISMiC were prospectively combined for analysis. In the analysis of Part B, all istaroxime-treated patients were compared to the placebo group. For certain endpoints in Part B, the dose-response between the two different istaroxime dose regimens was compared.
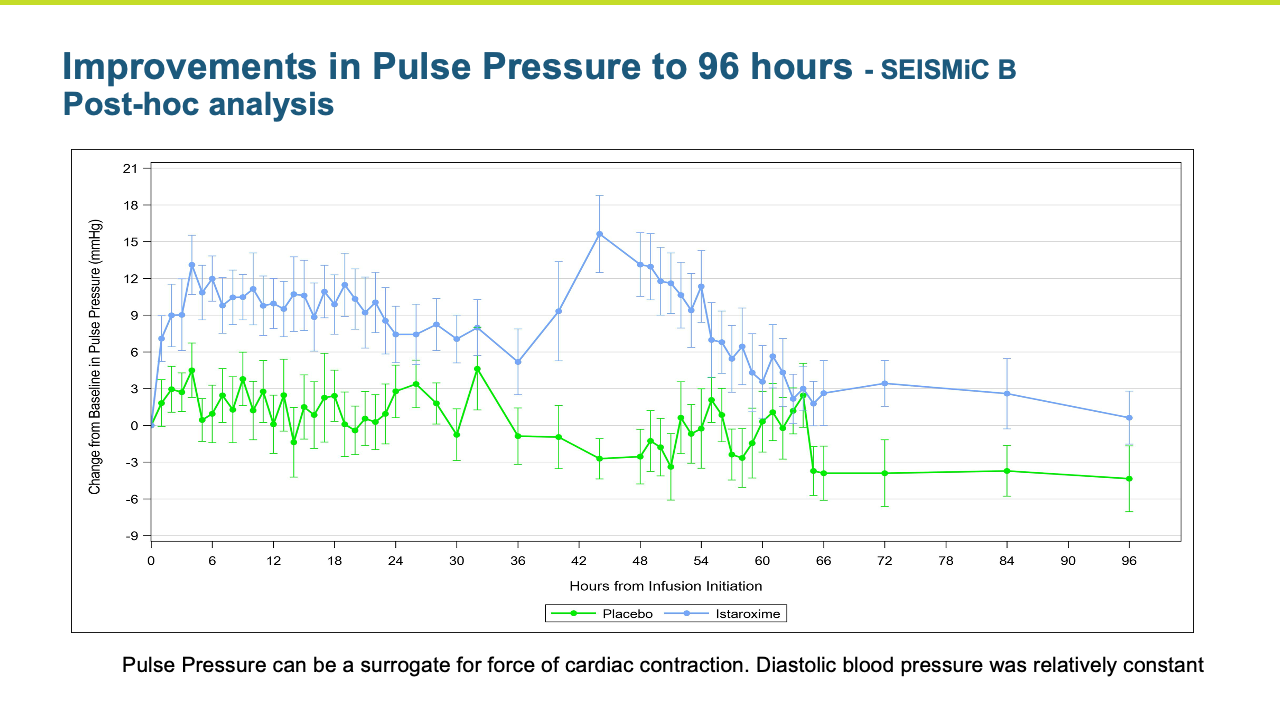
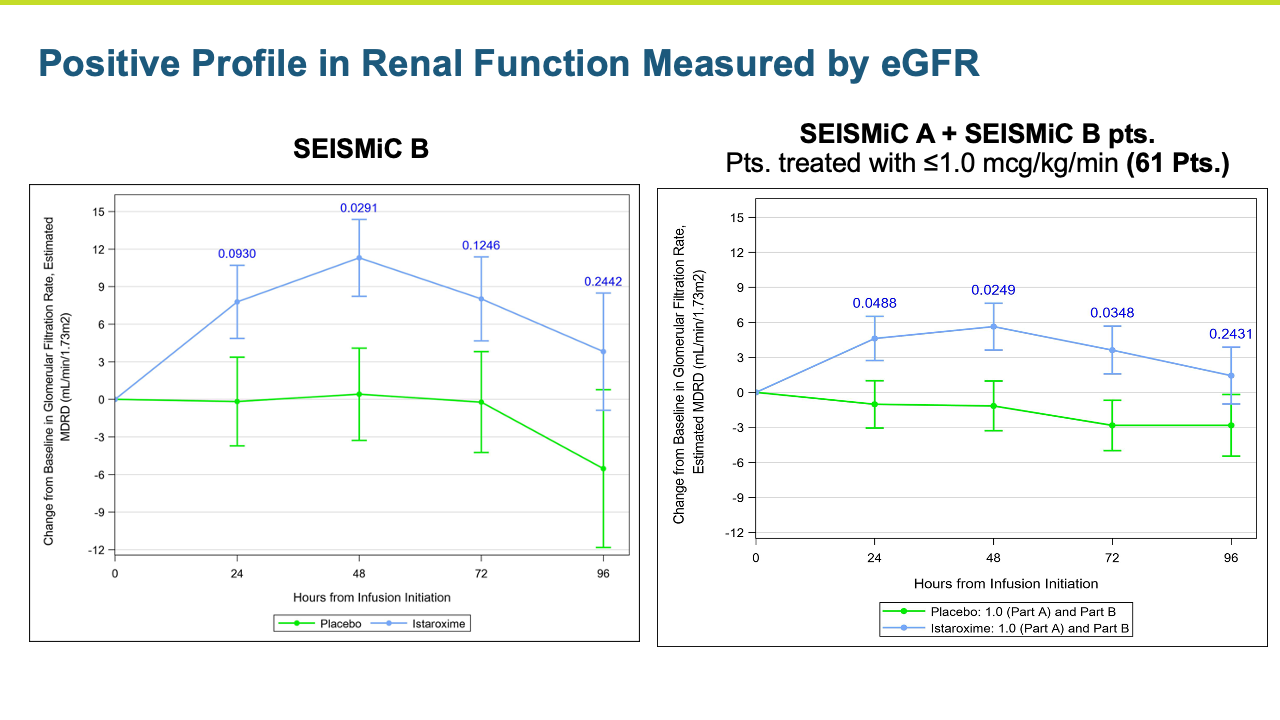
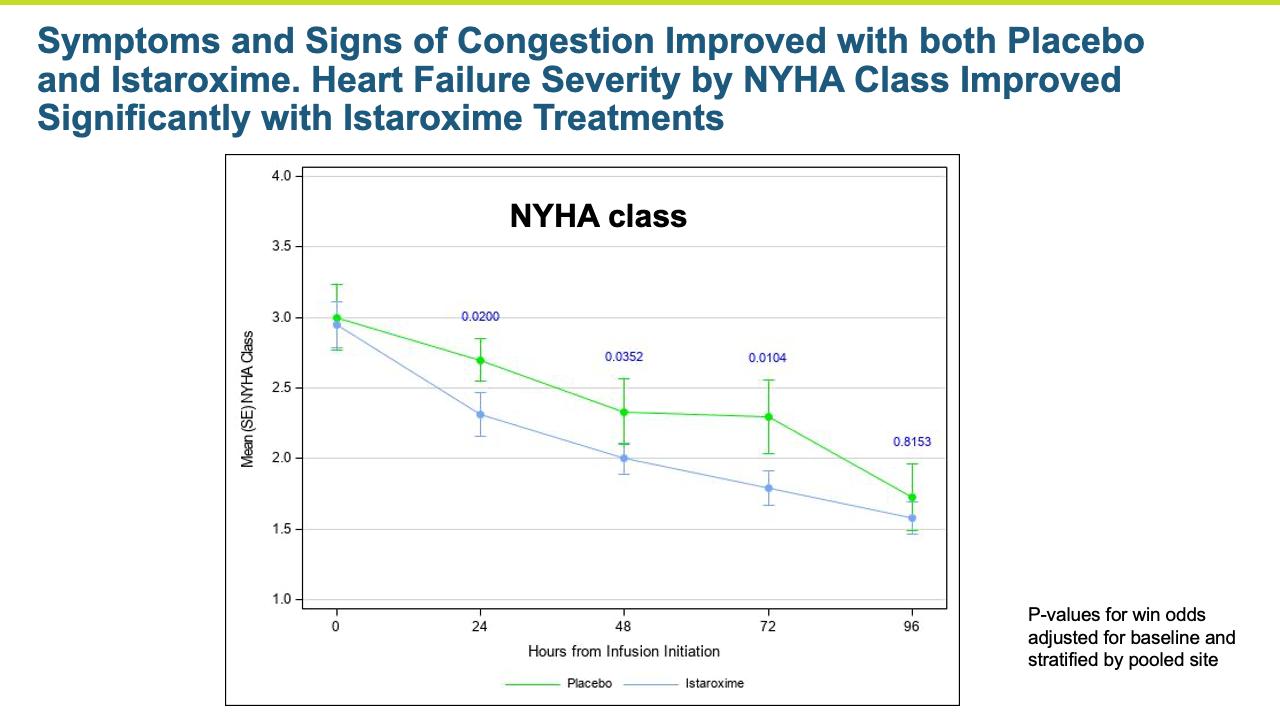
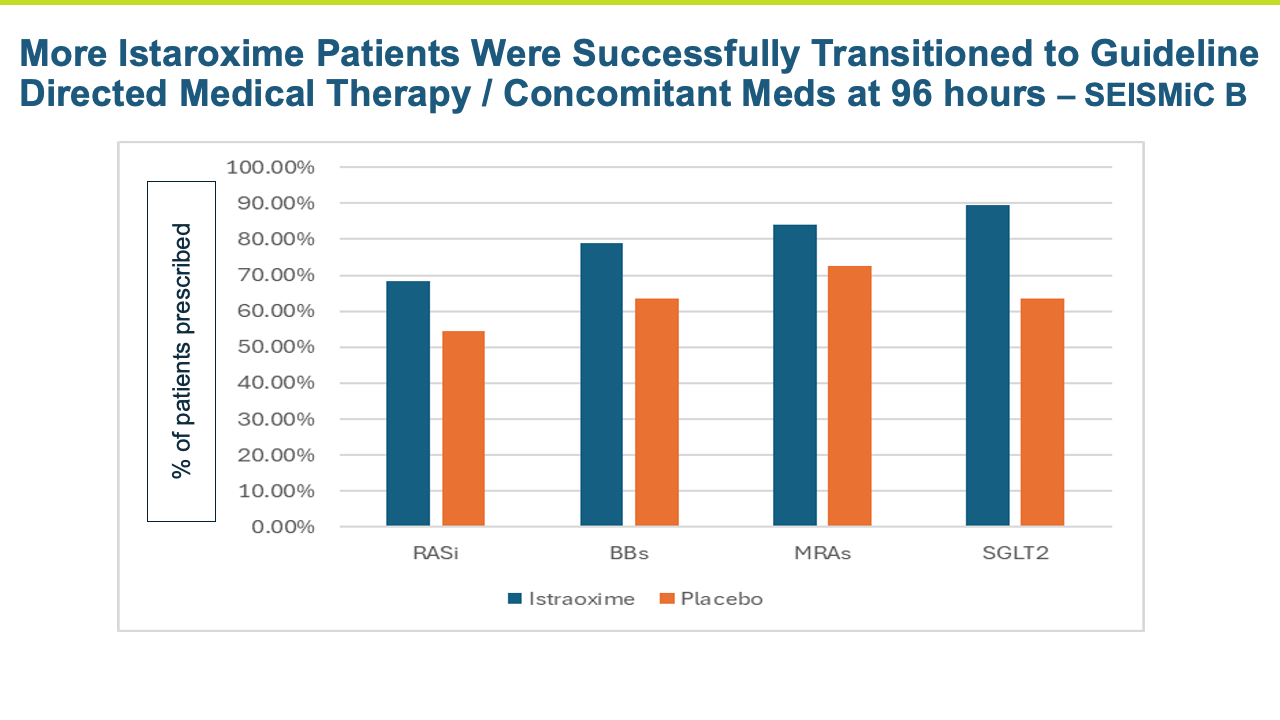
Heart failure represents a large number of patients with substantial unmet medical needs. There is a pronounced clinical and pharmacoeconomic burden and significant demand for alternative therapies.
The challenge of treating heart failure (HF) is well documented:
- One to two percent (1-2%) of the adult population have heart failure, including more than ten percent of all adults over the age of 65. The prevalence of heart failure is on the rise due, in part, to an aging population and increasing rates of diabetes and hypertension.1
- Heart failure is the leading cause of hospitalizations in the United States, with over 1,000,000 admissions annually. Inpatient mortality is seven percent (7%), whereas 30-day mortality exceeds ten percent (10%) and reaches 30 percent (30%) at 90 days.2
- Heart failure has a tremendous pharmacoeconomic cost. Estimates of the economic burden at $35B annually in the U.S. alone, with over half being direct hospital cost for acute treatment.3
- Heart failure is the most expensive of the Medicare diagnoses in the United States and yet, reimbursement often does not keep up with the mean total hospital charges, with the average American hospital losing money on each patient visit.4
Patients presenting to the hospital with ADHF are often complex and difficult to manage. Many patients have a history of arrythmias and issues with heart pacing. The Acute Decompensated Heart Failure National Registry (ADHERE) tracked over 104,000 patients and found 38 percent presented with low or below normal systolic blood pressure and in these patients, who have hypotension or evidence of inadequate organ perfusion, therapeutic options to increase myocardial performance are limited. This group of patients has longer length of stay in the hospital and are reported to more often be discharged in a sub-optimal cardiac condition.6 Another challenging group of patients are those with preserved ejection fraction (HFpEF), also referred to as diastolic heart failure, where the heart muscle contracts normally, but the ventricles do not relax as they should during ventricular filling. The proportion of HFpEF among all heart failure cases lies somewhere between 44 percent and 72 percent, with a suggestion of a temporal increase in the proportion of HFpEF cases in recent years.7
Several therapies are improving care of chronic heart failure in the outpatient, community setting, but effective treatments for acute, decompensated heart failure in the hospital are lacking. Patients who experience acute heart failure are generally placed on a strong IV diuretic such as furosemide. To augment cardiac output, physicians will sometimes use a drug class called inotropes to increase cardiac output and improve physiological parameters. These short-term improvements, however, have not resulted in improved clinical outcomes. On the contrary, the use of inotropes to directly target the ventricular dysfunction that characterizes heart failure has generally proven counterproductive. These and other older agents (as well as others that have failed in development) have been associated with an unacceptable rate of adverse events including arrhythmias, hypotension, worse clinical outcomes and in some settings an increased risk of death. Although these drugs increase the amount of blood pumped from the heart (or stroke volume), this may occur at the expense of increasing myocardial oxygen demand in an already compromised heart. In summary, current approaches to acutely improve cardiac function (inotropes) are associated with potential unwanted effects including:
• Heart rhythm disturbances;
• Increased heart rate and myocardial oxygen demand;
• Decreased blood pressure;
• Potential damage to the heart muscle (increased troponin);
• Worsening renal function; and
• Mortality.
Treatment is needed to improve cardiac function without causing other adverse clinical outcomes.
Istaroxime is a first-in-class, dual action, agent in clinical development for the treatment of acute decompensated heart failure (ADHF). Istaroxime’s dual mechanism is designed to improve both systolic contractions of the heart as a potent positive inotropic agent that increases contractility through inhibition of Na+/K+-ATPase, as well as its diastolic myocardial relaxation through activation of the SERCA2a calcium pump on the sarcoplasmic reticulum.
Based on clinical findings to date, we believe istaroxime has the potential to deliver the desired clinical effects of significantly improving cardiac function in decompensated heart failure without the deleterious effects of decreasing blood pressure, increasing heart rate, myocardial damage, or increased risk of arrhythmias.
Istaroxime has been studied in two Phase 2 clinical trials to date. The HORIZON study, a Phase 2a trial, examined the effects of three different doses of istaroxime infused for 6 hours in 120 patients and produced statistically significant (p<0.05) improvements of the primary endpoint by all three doses of istaroxime. The primary endpoint was the lowering of pulmonary capillary wedge pressure (PCWP). The study also showed positive trending on secondary hemodynamic endpoints, which measured the increased systolic blood pressure and decreasing heart rate. The main side effects were vomiting (7.9 percent) and pain at the infusion site (5.6 percent).
The favorable effects on PCWP, blood pressure, heart rate as well as its safety profile provided the basis for the program to move into a Phase 2b trial. The trial was completed in 120 patients examining the effect of two doses of istaroxime vs. placebo with a longer infusion of 24 hours. The primary endpoint of cardiac function was significantly (p<0.05) improved by both doses of istaroxime. This assessment used non-invasive echocardiography with a single, central reader to measure the E/Ea ratio (reflecting changes in PCWP or left ventricular filing pressure). Stroke volume was substantially increased as well. As important as the efficacy assessments were, the toxicities and complications that seemed to be avoided during istaroxime infusion, including no signals of increased clinically significant arrhythmias and no signals of increased cardiac troponins (a marker of heart muscle damage). Additionally, heart rate decreased, and blood pressure increased during the infusion supporting a positive profile on kidney function (important for effective diuretic therapy). This study replicated the physiologic improvements seen in the Phase 2a study and provides added evidence for continued development of istaroxime in patients with acute decompensated heart failure.
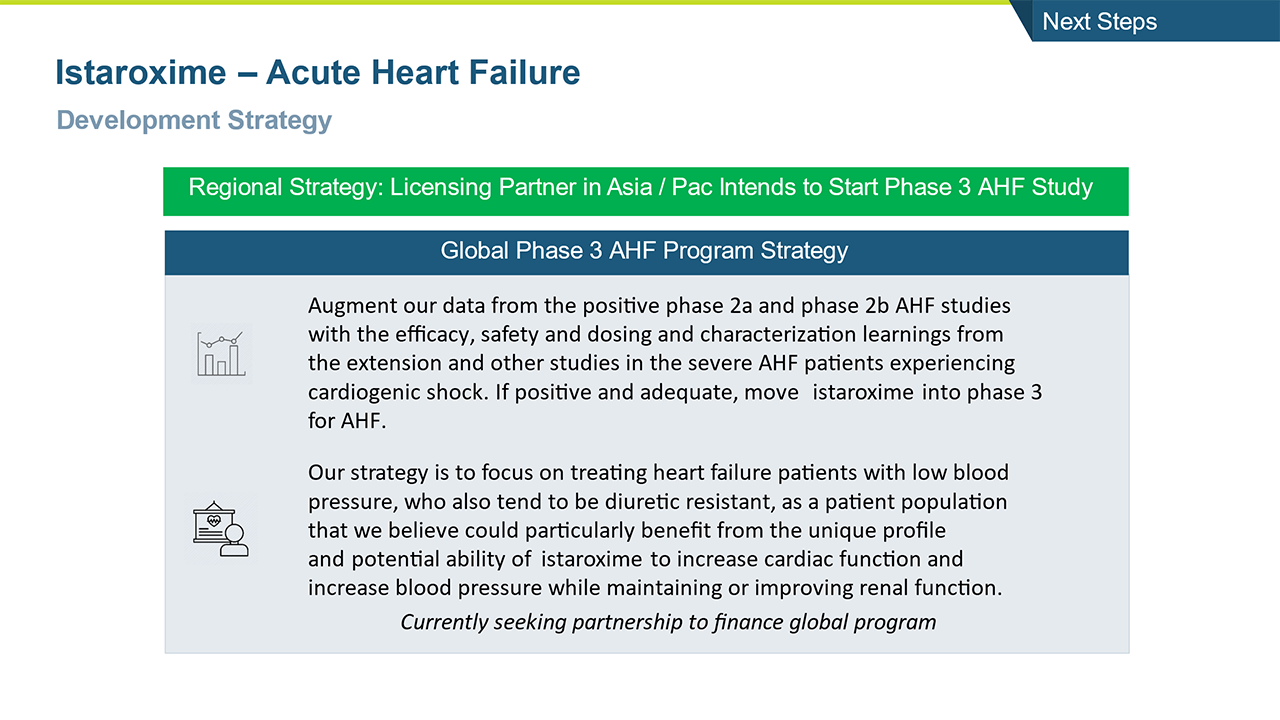
Istaroxime potential Phase 3 AHF program
- American Heart Association and references in the 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure
- Abraham et al, In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications. An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). Am J Cardiol 2005. 46:57-64
- Data from American Heart Association
- Peacock 2003; Ashish et al. 2004
- Ho, E. C., Schull, M. J., & Lee, D. S. (2012). The challenge of heart failure discharge from the emergency department. Current heart failure reports, 9(3), 252–259. https://doi.org/10.1007/s11897-012-0100-1
- Becher, P. M., Fluschnik, N., Blankenberg, S., & Westermann, D. (2015). Challenging aspects of treatment strategies in heart failure with preserved ejection fraction: “Why did recent clinical trials fail?”. World journal of cardiology, 7(9), 544–554. https://doi.org/10.4330/wjc.v7.i9.544
- Kolte D, American Heart Association; 2014 Jan 13
